Evaluation of the contact surface between the vertebral endplate and the 3D printed patient-specific cage compared to the commercial cage
Following Research Ethics Board approval (Western University Health Research Ethics Board #115303), five whole specimens (n=5) of unidentified spine cadavers were purchased by through a donor organization that provides cadavers for medical research and education. All procedures performed during the study complied with institutional guidelines and regulations for cadaveric and biomechanical studies in accordance with recommended safety protocols. Informed consent authorizing the use of the patient’s body or cadaveric specimen for educational, research and scientific purposes has been obtained from the next of kin by the donating organization.
The specimens were subjected to a CT-Scan to exclude bone tumors or fractures and obtain image acquisition for the segmentation process. Samples were kept frozen and soft tissues remained intact during the scanning process to replicate the clinical scenario as much as possible.
Then spines were isolated from L1 to L5 and prepared in the same way as described in previous studies15. A combination of gentle and sharp dissection using a scalpel, curettes, and periosteal elevators was used to remove soft tissue. We took special care when removing the cartilaginous plate to avoid damaging the underlying bony plate. After removing the soft tissues, the bones were dried at room temperature and embedded in cement with the cranial plate parallel to the ground (Fig. 1).
L5 vertebra potted in cement for biomechanical testing.
Bone segmentation
Open-source software, 3D Slicer (version 4.10.2), was used to create the 3D mesh models of the vertebrae by importing the digital CT imaging and communication in medicine (DICOM) files.
A region of interest was created around the lumbar spine (L1–L5), using data from the 0.625 mm slice thickness bone reconstructions. Each endplate model was created using semi-automatic segmentation by the “grow from seeds” extension in 3D Slicer’s segment editor (Fig. 2). Bones and soft tissues were extracted based on different Hounsfield Units (HU). Segmentation defects were corrected by modifying the seeds if necessary, taking care to compare the final segmentation model to the original CT-scan reconstruction. Additionally, a 2mm outer shell reflecting the contour of the vertebrae was created to allow the vertebrae to be used as guides to place the cages in the desired position during testing (Fig. 1).
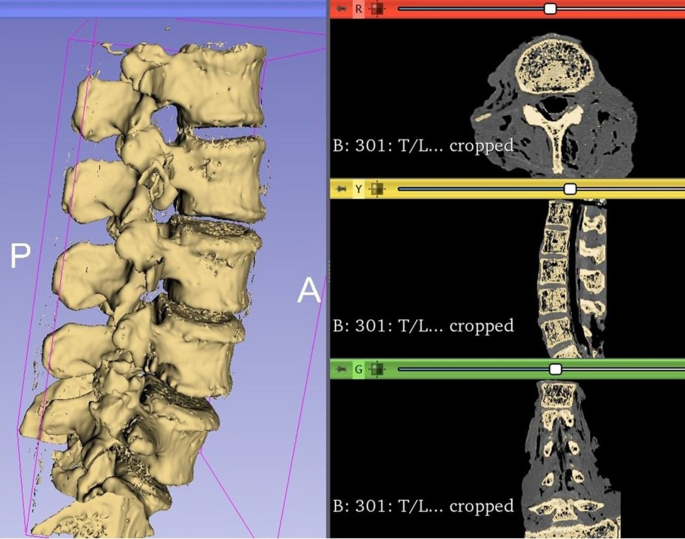
Example of 3D segmentation of the lumbar spine using 3D Slicer. Each vertebra file was saved as an individual file.
Choice of commercially available LIF cage models
We used two types of commercially available intervertebral LIF cages provided by a single leading medical device vendor (Medtronic Sofamor Danek USA, Inc., Memphis, TN, USA). One was made of titanium alloy and had a cylindrical shape (Fuse Spinal System); the other was made of PEEK and had a rectangular shape (Capstone PEEK Spinal System) (Fig. 3).
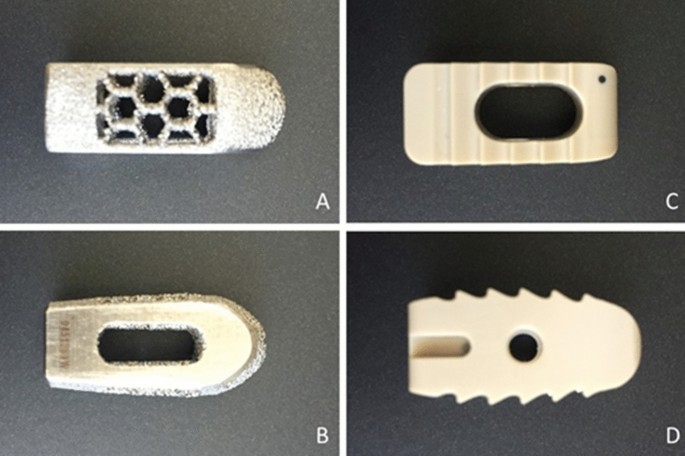
Top and side views of the fuse cage (A B) and capstone cage (CD).
Reverse engineering processes were used to replicate the dimensions and features of commercial cages. Modified implant models for slump testing were designed in 3D CAD modeling software (SolidWorks 2019, Dassault Systemes Solidworks Corp.). They were originally designed as a complete implant and then cut in half to allow the addition of a support base to secure the implant to the trial machine (Fig. 4), and the cylindrical cage model and the corresponding rectangular cage model files have been exported as STL file.
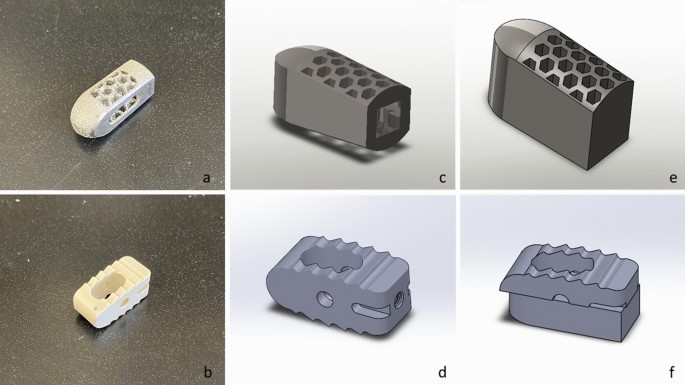
Images showing photos of the original Fuse and Capstone cages (a B), their complete implant CAD models (CD) and CAD models used for biomechanical testing (f,g).
Custom cages
The vertebra models and the cylindrical cage model were imported into STL editing software (Netfabb, Autodesk Inc, San Rafael, CA). Two replicate commercial implants (left and right) were translated for each endplate until their geometry overlapped with the upper endplate of each vertebra in a position similar to that in which it would be placed during an LIF surgery, thus determining the area where they would be tested in the cadaveric bone. After that, a Boolean subtraction operation was performed to create two patient-specific LIF cages per vertebra and the endplate guides (Figs. 1 and 5).
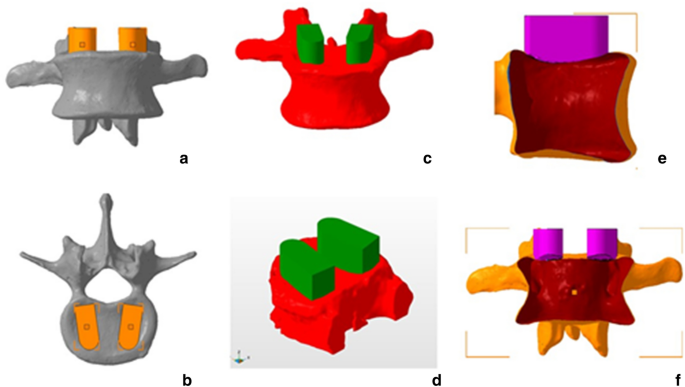
Anterior and superior views of planned cage positions(a B), forward and oblique views of the boolean(CD), and lateral and anterior views of the hollow vertebra and post-Boolean conformal implant operation (e,f).
3D printing of cages
All STL files, including patient-specific cages and modified cylindrical and rectangular shaped cages, were imported into FormLabs PreForm software for printing using a Form 2 printer (FormLabs, Somerville, MA) . The layer thickness was set at 50 microns to improve resolution. To standardize the material, all models were printed in rigid resin (FormLabs, Somerville, Massachusetts) reinforced with fiberglass making it resistant to deformation and having an elastic modulus similar to PEEK.
Eighteen of the 25 available vertebrae were used for testing, a sample size similar to previously published papers using pressure-sensitive film16.17. Four vertebrae were excluded from the 25 dissected lumbar vertebrae because they were damaged during the cadaver removal process. Three other vertebrae were excluded, one due to an anterior fracture, another due to Schmorl’s nodes and a third was damaged during potting. The 18 vertebrae were then divided into two groups of 9 vertebrae each: Group 1 compared the patient-specific and replicated Capstone, and Group 2 compared the patient-specific and replicated Fuse. The left and right side of each vertebra were tested for the patient-specific cage and the replicated commercial cage allowing 18 samples per comparison group.
Setting up the tests
A pressure-sensitive measurement film (“Ultra super low” Fujifilm, Pressure Metrics, Whitehouse Station, NJ, USA) was inserted at the interface of each cage-vertebra construct. The sheets were cut into 30mm x 30mm squares. The Fujifilm indicator layer was placed on top of the endplate and the acid layer on top of the indicator layer (Fig. 6). 3D-printed guides were used to determine the ideal cage position (Fig. 1). Using an electromechanical testing machine (Instron 5967, Norwood, MA, USA), the cages were axially compressed on the vertebral endplate with a force of 100 Newtons(N) for 30 s to obtain a uniform coloring. The force of 100 N was chosen to avoid damage to the end plate. All cages were filled with bone graft to reproduce similar conditions in surgery. Finally, the endplate surface was carefully inspected after each test to assess any surface conditioning that might interfere with the next test.
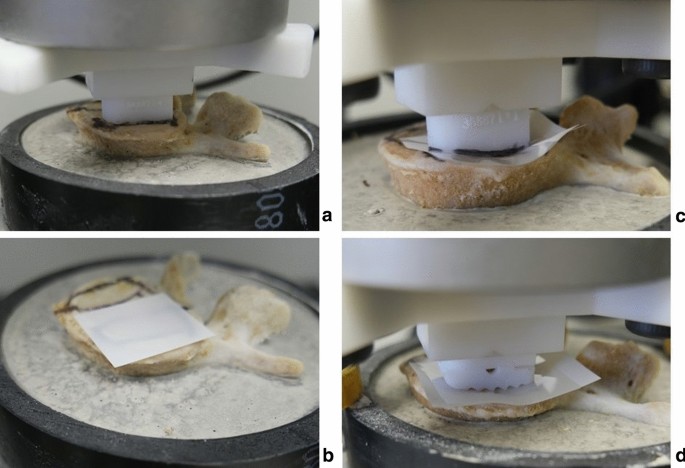
(a) The ideal cage position was determined using the PS cage, (b) FujiFilm was set up, (CD) PS and compressed commercial cage on the FujiFilm.
Analysis of contact area and contact stresses
After the load was removed, the Fujifilm sheets were carefully removed from the top of the end plate. Next, the contact areas of the Fujifilm indicator layers were scanned in jpeg format at 1200 dpi (dots per inch) using a desktop scanner (Hewlett-Packard, HP ENVY 4520) (Fig. 7). The maximum contact area of the cages that touched the endplates was calculated using ImageJ software (version 1.52, US National Institutes of Health, Bethesda, Maryland, USA). The average contact stress was obtained by dividing the applied force (100 N) by the measured contact surface and was expressed in megapascals (MPa).
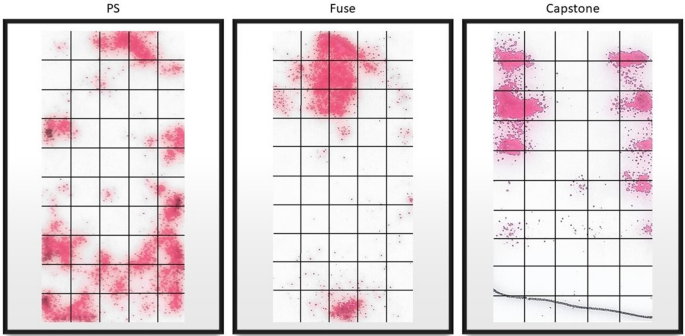
Example of imaging of the contact zone for each of the cages.
statistical analyzes
All statistical analyzes were performed using IBM SPSS version 26 (IBM Corp., Armonk, New York, USA). The comparison between the cages of each group was carried out using the Mann-Whitney U test. Statistical significance was set at p
Research ethics approval
#115303, Western University Health Research Ethics Board.


Comments are closed.